In Vitro Kinetic of Glutathione-S-Transferase Enzyme in (Buffalo, Camel, Sheep, Goat, and Cattle) and Inhibition by Aspirin and Paracetamol
DOI:
https://doi.org/10.59675/V225Abstract
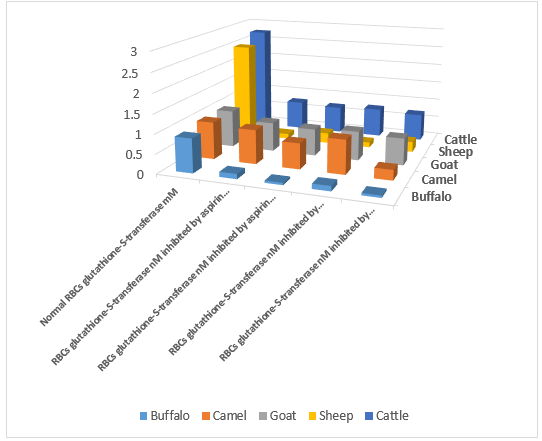
Enzyme kinetic showed competitive inhibition pathways for both aspirin and paracetamol. The activity of glutathione-s-transferase of RBCs (buffalo, camel, sheep, goat, and cattle) was measured by the photometric method in the presence and absence of two NSDI analgesic drugs (aspirin and paracetamol). Two different concentrations (10 and 20 mM) of each analgesic drug were used, which covered the reported therapeutic index and toxic concentration range of the drugs. At a level of significance (p < 0.05)
References
Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88.
Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J. 2001;360:1–16.
Allocati N, Federici L, Masulli M, Di Ilio C. Glutathione transferases in bacteria. FEBS J. 2009;276:58–75.
Allocati N, Federici L, Masulli M, Di Ilio C. Distribution of glutathione transferases in Gram-positive bacteria and Archaea. Biochimie. 2012;94:588–96.
Wu B, Dong D. Human cytosolic glutathione transferases: structure, function, and drug discovery. Trends Pharmacol Sci. 2012;33:656–68.
Oakley A. Glutathione transferases: a structural perspective. Drug Metab Rev. 2011;43:138–51.
American Society of Health-System Pharmacists. Aspirin. 29 November 2021. Archived from the original on 25 April 2017.
Katzung G. Basic and Clinical Pharmacology. 8th ed. New York: McGraw-Hill; 2001. p. 600–2.
Hardman JG, Limbird LE. Goodman and Gilman's The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 1996. p. 625–32.
Goodman LS, Gilman A. Pharmacological Basis of Therapeutics. New York: Macmillan Publishing Company; 1988.
Harwaldt P, Rahlfs S, Becker K. Glutathione S-transferase of the malaria parasite Plasmodium falciparum: characterization of a potential drug target. Biol Chem. 2002;383:821–30.
Neupane DP, Majhi S, Chandra L, Rijal S, Baral N. Erythrocyte glutathione status in human visceral Leishmaniasis. Indian J Clin Biochem. 2008;23(1):95–7.
Naqi MT. Toxicological effect of herbicide 2, 4-D (2,4-Dichlorophenoxyacetic acid) in the embryonic development parameters in chicken [MSc thesis]. Mosul: University of Mosul; 2006.
Sohail M, Kaul A, Raziuddin M, Adak T. Decreased glutathione S-transferase activity: diagnostic and protective role in vivax malaria. Clin Biochem. 2007;40(5–6):377–82.
Ayalogu EO, Igboh NH, Dede EB. Biochemical changes in the liver of albino rats exposed to petroleum samples (gasoline, kerosene, and crude petroleum). J Appl Sci Environ Manag. 2001;5(1):97–100.
Kamisaka K, Habig WH, Kelley JN, Arias LM, Jacoby WB. Multiple forms of human glutathione-S-transferase and their affinity for bilirubin. Eur J Biochem. 1975;60:153–61.
Burgen ASV, Mitchel JF. Gaddum's Pharmacology. 8th ed. Britain: Oxford University Press; 1978. p. 132–6.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Academic International Journal of Veterinary Medicine

This work is licensed under a Creative Commons Attribution 4.0 International License.