Pharmacokinetic Profile of Ceftriaxone and Meropenem in Dogs
DOI:
https://doi.org/10.59675/V224Abstract
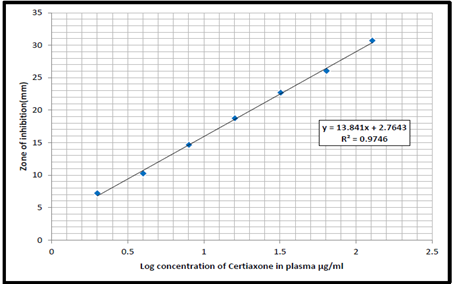
The principal aim of the study was evaluating the pharmacokinetic of ceftriaxone and meropenem in dogs, eight healthy male dogs were used for this experiment. A microbiological assay was used to determine the pharmacokinetic indices of ceftriaxone and meropenem given intravenously. The values were then fitted to a two-compartment pharmacokinetic open model in order to assess the factors related to distribution and excretion. The obtained results showed that the half-life, volume of distribution, and total body clearance to the samples of plasma of ceftriaxone and meropenem were recorded (0.83 h., 0.35 L/kg and 0.28 L/hr/kg), (0.86 h., 0.48 L/kg and 0.33 L/hr/kg), and the ratio of plasma protein binding were 16.67 %; 9.58 %, respectively.
In conclusion, through the pharmacokinetic characteristics of meropenem and ceftriaxone in dogs, they possess an efficacious profile against K. pneumonia as same as other sensitive bacteria which were qualified to be a potential candidate to be one of the most commonly used parenterally administered antibacterial medicines in the treatment of acute bacterial cases that need to be treated quickly in veterinary therapy. However, the differences in the pharmacokinetic profile proved that the effectiveness of meropenem was more than ceftriaxone.
References
Krunal S, Abhi D, Milind D, Harsh J. Review on important diseases of Dogs: At glance. Internationa Journal of Veterinary sciences and Animal Husbandry. 2023; 8(2):01-07.
Jonathan DD, Sean EH, Lynelle RJ. Recognition and Diagnosis of Underlying Disease Processes in Bacterial Pneumonia. Animals. 2024; 14(11): 1-11.
Jondle C N, Gupta K, Mishra BB and Sharma J. Klebsiella pneumoniae infection of murine neutrophils impairs their efferocytic clearance by modulating cell death machinery. PLoS. Pathogens. 2018; 14(10).
Mohammed MS, Orooba MSI. Pharmacodynamics analysis of meropenem against K. pneumonia isolated from COVID-19 patient. Biochem. Cell. Arch. 2022; 22 (1): 1777-1783.
Frei CR, Attridge RT, Mortensen EM, et al. Guideline-concordant antibiotic use and survival among patients with community-acquired pneumonia admitted to the intensive care unit. Clinical Therapeutics. 2010; 32(2): 293–299.
Khan MY, Roy M, Rawal RK.et al. A review-ceftriaxone for life. Asian Journal of Pharmaceutical Research. 2017; 7(1): 34-48.
Arumugham VB, Cascella M. Third Generation Cephalosporins. InStatPearls. 2019; 26:33-36.
Bassetti M, Peghin M, Vena A, et al. Treatment of infections due to MDR Gram-negative bacteria. Frontiers in medicine. 2019; 6: 74.
Levison ME, Levison JH. Pharmacokinetics and Pharmacodynamics of Antibacterial Agents. Infectious Disease Clinics of North America. 2009; 23: 791–815.
Sarhan RS, Orooba MSI, Bassam AAl. Study the pharmacokinetic of the Staphylococcus aureus specific bacteriophage in healthy animals. Journal of Entomology and Zoology Studies. 2017; 5(6): 170-176
Li H, Gao H, Meng H, et al. Detection of pulmonary infectious pathogens from lung biopsy tissues by metagenomic nextgeneration sequencing. Frontiers in cellular and infection microbiology. 2018; 8: 205.
Tamatsukuri T, Ohbayashi M, Kohyama N, et al. The exploration of population pharmacokinetic model for meropenem in augmented renal clearance and investigation of optimum setting of dose. Journal of Infection and Chemotherapy. 2018; 24(10): 834-840.
Craig WA. Protein binding and the antimicrobial effects: Methods for the determination of protein binding. Antibiotics in Laboratory Medicine. 1986; 477–514.
Rosenbaum SE. Basic Pharmacokinetics and Pharmacodynamics: An Integrated Textbook and Computer Simulations. John Wiley & Sons. 2016.
Yamaoka K, et al. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. Journal of Pharmacokinetics and Biopharmaceutics. 1978; 6: 165–175.
Ahmed T. Pharmacokinetics of drugs following IV Bolus, IV infusion, and oral administration. Basic Pharmacokinetic Concepts and Some Clinical Applications. 2015; 10.
Saunders LJ, Russell RA, Crabb DP. The coefficient of determination: what determines a useful R2 statistic?. Investigative ophthalmology and visual science. 2012; 53(11): 6830-6832.
Hornish RE, Katarski SF. Cephalosporins in veterinary medicine ceftiofur use in food animals. Current topics in medicinal chemistry. 2002; 2(7):717-731.
Adnan S, Paterson DL, Lipman J, Kumar S., LiJ., Rudd M, et al. Pharmacokinetics of beta-lactam antibiotics in patients with intra-abdominal disease: a structured review. Surg Infect. 2012; 13(1): 9-17.
Kanji S, Hayes M, Ling A. et al. Reporting guidelines for clinical pharmacokinetic studies: the Clin PK statement. Clinical pharmacokinetics. (2015; 54(7): 783-795.
Sandulovici R, Prasacu I, Mircioiu C, et al. Mathematical and phenomenological criteria in selection of pharmacokinetic model for M1 metabolite of pentoxyphylline. Farmacia. 2009; 57(2):235-247.
AL-Jumalli MAJ, Ibrahim OMS. Pharmacokinetic Parameters of Meropenem in the Plasma and Milk of Ewes. Indian Jl of Forensic Med Toxicol. 2021; 15: 8p.
Gabrielsson JL, Weiner DL. Methodology for pharmacokinetic /pharmacodynamic data analysis. Pharmaceutical science and technology today. 1999; 2(6): 244-252.
Ulldemolins M, Roberts JA, Rello J, et al. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clinical pharmacokinetics. 2011; 50(2): 99-110
Kok-Yong S, Lawrence L. Drug distribution and drug elimination. Basic pharmacokinetic concepts and some clinical applications. TA Ahmed. Rijeka, InTech. 2015; 99-116.
Yates JW, Arundel PA. On the volume of distribution at steady state and its relationship with two‐compartmental models. Journal of pharmaceutical sciences. 2008; 97(1):111-122.
Zhivkova Z. Quantitative structure–pharmacokinetics relationship for the steady-state volume of distribution of basic and neutral drugs. World J Pharm Pharm Sci. 2018; 7: 94-105.
Bidgood T, Papich MG. (2008). Plasma pharmacokinetics and tissue fluid concentrations of meropenem after intravenous and subcutaneous administration in dogs. American journal of veterinary research. 2008; 63(12): 1622-1628.
Reagan KL, Sykes J.E. Canine Infectious Respiratory Disease. The Veterinary clinics of North America. Small animal practice. 2020; 50(2): 405–418.
Baggot JD. Pharmacokinetic terms: symbols and units. Journal of veterinary pharmacology and therapeutics. 2001; 24(2): 81-82.
Nibras NA, Mustafa AAl, Orooba MSI. Pharmacokinetic Profile of Norfloxacin in Pigeons. Revista de Ciências Agroveterinárias. 2023; 22 (3): 470-474.
Tomaselli F, Maier A, Matzi V, et al. Penetration of meropenem into pneumonic human lung tissue as measured by in vivo microdialysis. Antimicrobial agents and chemotherapy. 2004; 48(6): 2228–2232.
Baldwin CM, Lyseng - Williamson K A, & Keam SJ Meropenem. Drugs. 2008; 68 (6): 803 - 838.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Academic International Journal of Veterinary Medicine

This work is licensed under a Creative Commons Attribution 4.0 International License.