Dexamethasone And Certain Nonsteroidal Drugs Stimulate The Transcriptional Activity Of GR-In The U2-OS Cancer Cell Line
DOI:
https://doi.org/10.59675/V222Abstract
Glucocorticoids are steroid hormones that act as immunosuppressants, immunomodifiers, and anti-inflammatory drugs. A synthetic GC chemical called dexamethasone (Dex) is used for a number of things other than reducing inflammation. Dex stops cell proliferation in leukemia and other cancers. In order to help chemotherapy kill leukemia cells or to minimize side effects from specific chemotherapy drugs, steroids are frequently given along with chemotherapy. The two steroids that are most frequently administered for ALL are prednisolone and dexamethasone. Steroid pills are typically taken. In both people and animals, chronic GC use increases the risk of illnesses in the nephrological system, skeletal system, and metabolism.
The current study sought for strategies to improve the current glucocorticoid medication in order to treat leukemia. The effects of numerous substances on cells were examined using a variety of procedures.
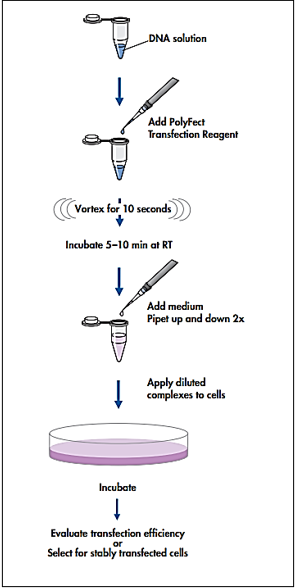
This study investigated the dissociation effects of CPDA TYRAMINE AND THCL to dexamethasone on wild GR and transfected Mutant GR-I628A using the cancer cell line U2-OS and the luciferase assay. The results show that DEX stimulates the transcriptional activity of wild GR. Following Dex treatment, TAT3 luciferase reporter activity was elevated in both mutant and wild-type GR.
The other non-steroidal medications that were tested also demonstrated positive regulation activity, with DEX, T, CpdA, and THCL upregulating TAT3 transcription, in that order. Given that transactivation activity is the only adverse consequence, this implies that other drugs may be less likely to do so than GR. Additionally, residue I628 appears to be essential for interaction because its mutation decreases GR activity when it interacts with all drugs.
References
Brownstein, M. J. Adrenocorticotropic hormone (ACTH) in the central nervous system. Advances in biochemical psychopharmacology, (1980), 22, 93–99.
COLE, T. J., Short, K. L., & Hooper, S. B.. The science of steroids. Seminars in fetal & neonatal medicine, (2019),24(3), 170–175. https://doi.org/10.1016/j.siny.2019.05.005
DARE, JB, Arogundade B, Awoniyi OO, Adegoke AA, Adekomi DA. Dexamethasone as endocrine disruptor; type I and type II (anti) oestrogenic actions on the ovary and uterus of adult Wistar rats (Rattus Novergicus). JBRA Assist Reprod. 2018 Nov 1;22(4):307-313. doi: 10.5935/1518-0557.20180061. PMID: 30175909; PMCID: PMC6210616.
DE WET, J. R., Wood, K. V., DeLuca, M., Helinski, D. R., & Subramani, S. Firefly luciferase gene: structure and expression in mammalian cells. Molecular and cellular biology, (1987). 7(2), 725–737. https://doi.org/10.1128/mcb.7.2.725-737.1987
FRANK, F., Ortlund, E. A., & Liu, X.. Structural insights into glucocorticoid receptor function. Biochemical Society transactions, (2021) 49(5), 2333–2343. https://doi.org/10.1042/BST20210419
HAGYMASI, A. T., Dempsey, J. P., & Srivastava, P. K. Heat-Shock Proteins. Current protocols, (2022). 2(11), e592. https://doi.org/10.1002/cpz1.592
HEROLD, M. J., McPherson, K. G., & Reichardt, H. M. Glucocorticoids in T cell apoptosis and function. Cellular and molecular life sciences : CMLS, (2006). 63(1), 60–72. https://doi.org/10.1007/s00018-005-5390-y
ITAHANA, K., Campisi, J., & Dimri, G. P. Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods in molecular biology (Clifton, N.J.), (2007). 371, 21–31. https://doi.org/10.1007/978-1-59745-361-5_3
KADMIEL, M., & Cidlowski, J. A. Glucocorticoid receptor signaling in health and disease. Trends in pharmacological sciences, (2013), 34(9), 518–530. https://doi.org/10.1016/j.tips.2013.07.003
LIN, Y., Zhang, Z., Wang, S., Cai, J., & Guo, J. Hypothalamus-pituitary-adrenal Axis in Glucolipid metabolic disorders. Reviews in endocrine & metabolic disorders, (2020).21(4), 421–429. https://doi.org/10.1007/s11154-020-09586-1
McColl, A., Michlewska, S., Dransfield, I., & Rossi, A. G. Effects of glucocorticoids on apoptosis and clearance of apoptotic cells. TheScientificWorldJournal, (2007), 7, 1165–1181. https://doi.org/10.1100/tsw.2007.224
MUN, E. J., Babiker, H. M., Weinberg, U., Kirson, E. D., & Von Hoff, D. D.. Tumor-Treating Fields: A Fourth Modality in Cancer Treatment. Clinical cancer research : an official journal of the American Association for Cancer Research, (2018), 24(2), 266–275. https://doi.org/10.1158/1078-0432.CCR-17-1117
SUNDAHL, N., Bridelance, J., Libert, C., De Bosscher, K., & Beck, I. M.. Selective glucocorticoid receptor modulation: New directions with non-steroidal scaffolds. Pharmacology & therapeutics, (2015), 152, 28–41. https://doi.org/10.1016/j.pharmthera.2015.05.001
SUSKE G. .Transient transfection of Schneider cells in the study of transcription factors. Methods in molecular biology (Clifton, N.J.), (2000). 130, 175–187. https://doi.org/10.1385/1-59259-686-x:175
WATERS, C. E., Stevens, A., White, A., & Ray, D. W.. Analysis of co-factor function in a glucocorticoid-resistant small cell carcinoma cell line. The Journal of endocrinology, (2004), 183(2), 375–383. https://doi.org/10.1677/joe.1.05804
ZHANG, T., Liang, Y., & Zhang, J.. Natural and synthetic compounds as dissociated agonists of glucocorticoid receptor. Pharmacological research, (2020), 156, 104802. https://doi.org/10.1016/j.phrs.2020.104802
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Academic International Journal of Veterinary Medicine

This work is licensed under a Creative Commons Attribution 4.0 International License.